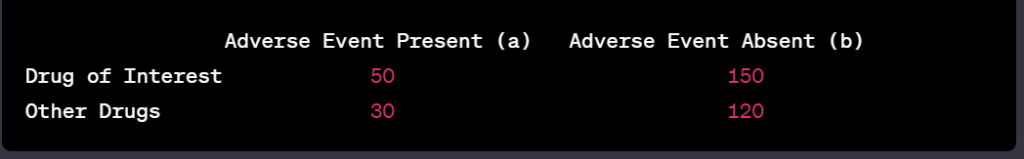
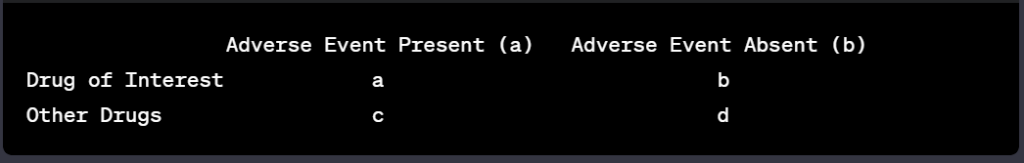
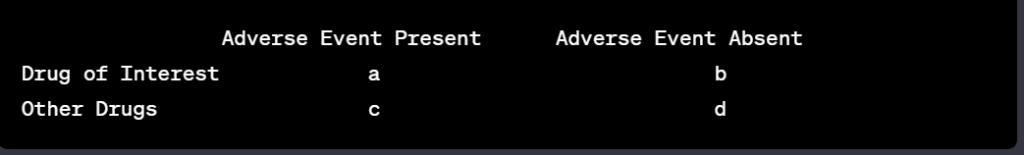
Artificial Intelligence (AI) has emerged as a transformative force in the realm of drug safety monitoring, guiding stakeholders through the meticulous landscape of pharmacovigilance. This technology harnesses computational power and sophisticated algorithms to process and analyze vast quantities of data, aiming to predict, monitor, and prevent adverse drug reactions (ADRs). The integration of AI into this field marries the complexities of medical research with the precision of machine learning, offering a proactive approach to safety throughout the various stages of drug development.
The implementation of AI systems in drug safety monitoring not only streamlines the detection of ADRs but also enriches the drug development process by providing actionable insights during clinical trials. These technological advancements are pivotal in sifting through intricate data sets, ranging from patient health records to clinical trial data, and are instrumental in enhancing the decision-making process. By applying methods like natural language processing and deep learning, AI offers a valuable lens through which safety signals can be identified more efficiently, reducing the potential risk to patients and accelerating time to market for new medications.
Key Takeaways
- AI revolutionizes drug safety by improving the detection and analysis of adverse reactions.
- Enhanced decision-making in clinical trials is facilitated by AI’s deep learning capabilities.
- Streamlined pharmacovigilance is achieved through AI’s management and interpretation of complex data.
Fundamentals of AI for Drug Safety
Artificial intelligence has revolutionized the landscape of drug safety, where machine learning and deep learning techniques are increasingly integrated to predict, monitor, and manage adverse drug reactions efficiently.
Defining AI and Machine Learning
Artificial Intelligence (AI) refers to the simulation of human intelligence in machines that are programmed to think and learn. Machine learning (ML), a subset of AI, employs algorithms to parse data, learn from that data, and then make informed decisions based on what it has learned. Deep learning, a more specific subset of ML, uses neural networks with several layers to analyze complex patterns in large datasets.
Role of AI in Healthcare
In the realm of healthcare, AI is positioned to enhance a variety of applications, specifically in advancing drug safety monitoring. AI-driven tools can swiftly analyze vast volumes of medical data to identify potential adverse reactions to medications, often with greater accuracy than traditional methods. These tools aid in the preclinical and clinical stages of drug development, as well as in post-marketing surveillance, to ensure medicine safety is upheld throughout a drug’s lifecycle.
Drug Development and Clinical Trials
The integration of Artificial Intelligence (AI), particularly deep learning technologies such as Convolutional Neural Networks (CNNs), has revolutionized drug development and clinical trials, improving efficiency and precision from the initial discovery phase through preclinical and clinical evaluations.
Innovations in Drug Discovery
Drug discovery has been transformed by AI, specifically through innovative approaches like de novo drug design, which allows for the creation of novel drugs that are optimized for safety and efficacy. Utilizing AI in drug discovery can significantly shorten the timeframe for developing new drugs and enhance the ability to predict how drugs will interact with biological systems.
Enhancing Clinical Trial Design
Clinical trial design benefits from AI by employing advanced algorithms to refine study parameters, leading to more effective and tailored clinical research. Data-driven methods, enabled by deep learning, help identify the ideal patient cohorts and can potentially predict trial outcomes, leading to a more streamlined path toward drug approval.
Preclinical Evaluations and Safety
In the realm of preclinical drug safety, the application of AI systems, such as CNNs, is pivotal for toxicity evaluation, improving the prediction of adverse effects before clinical trials begin. This preclinical evaluation is crucial in safeguarding patient safety and ensuring that the most promising compounds move forward in the drug development pipeline.
AI’s role in early-stage toxicity evaluation allows researchers to filter out compounds with potential safety issues, thereby reducing the risk of late-stage clinical trial failures.
Pharmacovigilance and Monitoring
Pharmacovigilance (PV) is integral for ensuring drug safety post-approval. Artificial Intelligence (AI) significantly enhances the detection and analysis of adverse drug reactions (ADRs), optimizing this critical healthcare aspect.
Basics of Pharmacovigilance
Pharmacovigilance (PV) is the science and activities concerned with the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem. The World Health Organization (WHO) Programme for International Drug Monitoring and regulatory bodies like the U.S. Food and Drug Administration (FDA) lead global efforts in PV. Post-marketing surveillance plays a pivotal role in this field as it involves monitoring the safety of pharmaceutical products after they have been released on the market.
- Objectives of PV:
- Protect patients from unnecessary harm.
- Provide up-to-date safety information.
- Promote the safe and effective use of medicines.
AI in Drug Safety Surveillance
The integration of AI into drug safety surveillance has transformed pharmacovigilance (PV). AI technologies can process large volumes of data and recognize patterns that might go unnoticed by humans. These potent data analysis capabilities are being applied to various PV activities, such as evaluating the safety data from clinical trials and generating new safety hypotheses from real-world evidence.
- Applications of AI in PV:
- Automating case processing: To expedite the review of individual case safety reports (ICSRs).
- Signal detection: Enhanced detection and management of potential safety signals from diverse data sources.
Detecting Adverse Drug Reactions
Adverse drug reactions (ADRs) pose a considerable health risk and are a significant focus for PV monitoring. AI aids in the timely identification of ADRs, supporting the FDA Adverse Event Reporting System and WHO’s spontaneous reporting mechanisms. AI algorithms can sift through vast datasets from electronic health records, scientific literature, and social media to detect potential adverse events earlier and more accurately than traditional methods.
- Improving ADR Detection:
- Pattern Recognition: Identifying trends in adverse events (AEs) that might indicate underlying safety issues.
- Predictive Analysis: Using historical data to predict the likelihood of future ADRs.
Data Sources and Management
Effective drug safety monitoring relies on diverse data sources and robust management practices. The integration of large datasets from electronic health records and dedicated drug safety databases plays a critical role, while emerging social media platforms offer a vast, untapped resource for real-time monitoring.
Leveraging Electronic Health Records
Electronic health records (EHRs) are a pivotal source of patient data. They provide comprehensive medical histories, which, when aggregated, offer valuable insights for drug safety analyses. EHR systems facilitate a data-driven approach to identify adverse drug reactions, leading to improved patient outcomes.
Drug Safety Databases
Several specialized databases such as DrugBank, ChEMBL, and the ExAC Browser have become indispensable in drug safety monitoring. These databases compile detailed drug properties, clinical trial results, and genetic information, aiding researchers in rapidly assessing potential safety issues.
Social Media as a Data Resource
Social media data is increasingly recognized for its role in pharmacovigilance. Social media platforms provide real-time, patient-reported information on drug effects, often capturing data outside of traditional healthcare settings. The challenge lies in parsing and analyzing this unstructured data to extract actionable safety signals.
AI Methodologies and Applications
In the realm of drug safety monitoring, AI methodologies are revolutionizing how data is analyzed and interpreted. These applications utilize powerful algorithms and models to improve the efficiency and efficacy of pharmacovigilance.
Machine Learning Techniques
Machine Learning (ML), a subset of AI, encompasses a variety of techniques that enable systems to learn from data, identify patterns, and make decisions. Bayesian techniques are particularly useful in drug safety due to their ability to manage uncertainty and incorporate prior knowledge into the model. Decision forest models, another form of machine learning algorithm, provide robust classification tools that help determine the safety profile of drugs by evaluating multiple data points simultaneously.
Natural Language Processing for PV
Natural Language Processing (NLP) stands critical in pharmacovigilance (PV) for its ability to process vast amounts of unstructured data, such as patient reports and clinical literature. By extracting relevant safety signals from text, NLP systems can vastly improve the identification and reporting of adverse drug reactions. Recent advancements have seen NLP being combined with other AI techniques to further enhance its applicability in drug safety monitoring.
Deep Learning Approaches
Deep Learning (DL) approaches, particularly convolutional neural networks (CNNs), are at the forefront of image-based data analysis, which can be applied in the evaluation of drug toxicity. Deep autoencoder neural networks are also showing promise in identifying complex, non-linear relationships within high-dimensional pharmacological data. Such deep learning models have the potential to uncover insights that might be missed by traditional analytical techniques, paving the way for a new era in drug safety evaluation.
Toxicity and Safety Evaluation
An integral component of drug development is ensuring the safe use of medications. This section details the methods through which artificial intelligence (AI) enhances the identification and analysis of potential drug toxicity and adverse reactions.
Predictive Modeling of Toxicity
Predictive modeling applies AI techniques such as quantitative structure-activity relationship (QSAR) and chemoinformatics to forecast the toxicity of new compounds. QSAR models correlate chemical structure with toxicity, allowing for the prediction of adverse drug reactions before clinical trials. Bayesian networks and genetic algorithm-multiple linear regressions offer sophisticated means to mine datasets and generate models that predict chemical toxicity with increasing accuracy.
Chemoinformatics tools are crucial in processing large chemical datasets to find patterns related to drug safety. Their application in predictive modeling enhances the speed and precision with which compounds are screened for potential toxicity.
Drug-Induced Organ Toxicity
Drug-induced liver injury (DILI) is a significant concern in pharmacology. AI assists in the early detection of compounds that might cause DILI, safeguarding patient health and reducing late-stage drug development failures. Identifying the markers of organ toxicity often involves complex analysis of biological data, where AI algorithms excel in pattern recognition.
Chemical databases and AI-driven methods are being continuously refined to better understand the toxicity mechanisms at play, leading to more reliable predictions of how drugs might affect organs like the liver or the heart. This precision is key in minimizing adverse drug reactions and ensuring the safety and efficacy of new medications.
Challenges and Future Directions
The pursuit of enhancing drug safety monitoring through artificial intelligence (AI) confronts several challenges and demands strategic directions. As AI integrates deeper into pharmacovigilance (PV), recognizing and addressing these issues is crucial for the pharmaceutical industry and public sector.
Addressing Multimorbidity and Drug-Drug Interactions
Multimorbidity, the presence of multiple chronic diseases in a patient, and drug-drug interactions represent significant hurdles for drug safety. As patients often take multiple medications, the risk of adverse drug reactions increases. AI must evolve to predict these interactions more accurately, considering the complexity of multimorbidity. Innovation in AI algorithms is crucial, as they must be trained on diverse data sets that include varied patient demographics and polypharmacy scenarios.
Advancement in Precision Medicine
The field of precision medicine is advancing rapidly, tailoring treatment based on an individual’s genetic profile and environmental factors. AI can aid in the interpretation of genotype-tissue expressions, enhancing the ability to predict individual responses to drugs. The pharmaceutical industry and public sector need to fund research to enable AI systems to utilize large databases, refining the accuracy of personalized treatment plans.
Dealing with Underreporting
Underreporting of adverse drug reactions is a persistent challenge in drug safety monitoring. It impedes the ability of healthcare professionals to assess the true risk profile of medications. AI systems could advance to pinpoint patterns that suggest underreporting and prompt further investigation. This ability would provide a broader view of drug safety, enhancing the experience professionals rely on for decision-making.
Leveraging Real-World Data for PV
Real-world data (RWD) — data gathered outside of controlled clinical trials — is a treasure trove for pharmacovigilance. AI can harness this data to observe drug performance in diverse populations across various healthcare settings. However, the challenge lies in how to effectively integrate and analyze such vast and unstructured datasets. With adequate funds and collaboration across the pharmaceutical industry and public sector, AI can be developed to systematically leverage RWD, leading to more innovative drug repurposing and safety monitoring strategies.
Regulatory and Ethical Considerations
As artificial intelligence (AI) becomes more prevalent in drug safety monitoring, regulatory frameworks and ethical considerations must adapt to ensure safety and efficacy. These frameworks guide the use of AI in making decisions that have far-reaching consequences on patient health and privacy. Strong oversight can reinforce trust and enable advancements in this field.
FDA and International Guidelines
The U.S. Food and Drug Administration (FDA) provides regulatory guidance for AI applications in medical products, aiming to balance innovation with patient safety. This involves oversight of AI technologies, from development to post-market surveillance, ensuring compliance with medical device regulations. The FDA’s approach is part of a broader international trend, where regulatory agencies like the World Health Organization (WHO) and the Uppsala Monitoring Centre (part of WHO’s program for International Drug Monitoring) set standards to maintain drug safety at a global scale. They ensure the ethical use of AI by collaborating with regulators worldwide, providing a framework to monitor adverse drug reactions and share best practices.
Ethical Implications of AI in Medicine
Ethical use of AI in medicine requires careful consideration of patient privacy, informed consent, and the avoidance of bias. AI systems must be transparent and accountable, as they process significant amounts of sensitive data while identifying patterns in drug safety and efficacy. The potential for AI to inadvertently perpetuate discrimination or ethical breaches is a substantial concern that calls for stringent ethical guidelines and ongoing oversight. The interplay of ethical frameworks with AI technologies must evolve continually to address novel challenges posed by advancements in machine learning and data analytics.
The Role of Public Sector and Funding
The public sector plays a pivotal role in supporting AI in drug safety monitoring through funds and resources, facilitating research, and enforcing regulations. Investments and funding from government entities drive the development of innovative AI applications, ensuring these technologies serve the public interest. Public sector agencies also collaborate with private entities to harmonize regulation and support large-scale data analysis efforts. Sufficient public funding encourages ethical research into AI’s potential and limitations, ensuring these technologies are developed and implemented responsibly.
Entities like the FDA, WHO, and the public sector are key in providing oversight and funding for AI’s safe and ethical use in drug safety monitoring.