In the field of healthcare, leveraging the power of predictive models to identify drug risks before they affect patients is becoming increasingly crucial. With many medications on the market and more entering it each year, the ability to predict adverse drug reactions can significantly enhance patient safety and therapeutic effectiveness. These models are built on a variety of statistical and machine learning techniques that can analyze extensive datasets to forecast potential drug risks.
This predictive capability is particularly important in the context of personalized medicine, where a patient’s unique characteristics, such as genetic makeup, can affect their response to drugs. Integrating diverse biomedical data sources, including electronic health records and genomic data, helps create more accurate and individualized risk profiles. As machine learning algorithms grow more sophisticated, they open new avenues in drug risk assessment, allowing researchers to uncover complex patterns and interactions that may not be apparent using traditional statistical methods.
Key Takeaways
- Predictive models facilitate early detection of drug risks, improving patient outcomes.
- Machine learning techniques enhance the precision of drug risk assessments.
- Integration of diverse data sources is pivotal for advancing personalized medicine.
Fundamentals of Predictive Modeling
Predictive modeling is a cornerstone in the realm of drug development, leveraging the power of both statistical analysis and machine learning techniques to anticipate drug risks.
Overview of Predictive Models
Predictive models are computational tools that project future events based on historical data. In the context of drug development, these models analyze patterns in clinical trial data to forecast potential adverse drug events. The efficacy of these models largely hinges on the data quality and the appropriateness of the statistical or machine learning techniques employed.
Importance in Drug Development
Within drug development, the application of predictive analytics is crucial for identifying FDA-approved drugs’ side effects that might not be evident during initial clinical trials. By efficiently signaling potential risks, predictive models can save pharmaceutical companies time and resources, while protecting patient safety.
Key Statistical and Machine Learning Techniques
Key techniques in predictive modeling encompass a wide range of statistical and machine learning approaches. Statistical analysis forms the basis for understanding data relationships, while machine learning techniques like natural language processing (NLP) and next-generation sequencing (NGS) parse through complex, unstructured data sets to identify drug risk. Within this landscape, feature selection is imperative to refine models, focusing on the most relevant predictors, such as patient phenotypes and genetic profiles acquired from high-throughput screening.
Challenges and Considerations
The accuracy of predictive models is contingent upon the volume and quality of the data. Factors like missing information, inaccuracies, and biased data sets can lead to underperforming models. Furthermore, while statistical models rely on pre-determined equations, machine learning models may require vast amounts of training data to learn and adapt. It is also crucial to address the interpretability of the model to ensure that the predictions can be understood and trusted by medical professionals.
Advancements in Computational Tools
The integration of advanced computational models has propelled the field forward. Developments in artificial intelligence, especially in areas such as natural language processing and machine learning, allow for the synthesis of large datasets from diverse sources like electronic health records and literature databases. This multi-faceted approach enriches predictive modeling by introducing broader context and facilitating deeper insights into drug safety and efficacy.
Machine Learning in Drug Risk Assessment
Machine learning plays a crucial role in enhancing drug risk assessment by providing sophisticated models that can predict drug response, identify potential risks, and streamline drug development.
Predictive Algorithms for Drug Development
Researchers utilize predictive algorithms for drug development to reduce risks and costs associated with clinical trials. These algorithms analyze vast datasets to forecast adverse reactions and efficacy, enabling a more targeted approach in early-stage research.
Machine Learning Approaches to Oncology
In the realm of precision oncology, machine learning approaches refine drug sensitivity prediction by incorporating global cancer statistics and omic profiles. They focus on identifying biomarkers that signal a tumor’s potential reaction to treatment, thus personalizing therapy for cancer patients.
Predictive Modelling for Precision Medicine
Predictive modelling has shown promise in precision medicine, particularly personalized medicine, where treatments are adapted to the individual’s genetic makeup. By analyzing cell line data and biomarkers, these models suggest optimal treatment strategies for the individual’s specific disease profile.
Multi-Task Learning and Network Approaches
Multi-task learning and complex neural network architectures contribute to a better understanding of drug responses. By learning from diverse but related tasks simultaneously, multi-task frameworks can discern subtle patterns across various types of drugs and diseases, leading to more reliable predictors of drug efficacy and toxicity.
Evaluating Drug Response and Sensitivity
Accurate evaluation of drug response and sensitivity is achieved through advanced machine learning algorithms that process comprehensive datasets. These algorithms enable the exploration of drug repurposing opportunities by identifying potential new uses for existing drugs based on drug response prediction analyses and have become essential tools in the development of targeted therapies.
Integrating Diverse Biomedical Data Sources
Successful predictive modeling in drug risk identification crucially depends on the integration of diverse biomedical data sources ranging from genetic information to clinical data. This complex convergence is geared towards understanding drug risks and patient-specific responses at a granular level.
Application of Omics Data in Predictive Modeling
Omics data, including gene expression and RNA-sequencing, offers a comprehensive view of an organism’s biological processes. Predictive models utilize omics profiles to identify patterns that may indicate adverse drug reactions. For instance, variations in gene expression can suggest how an individual might respond to a given drug, potentially reducing the risk of unanticipated effects.
Incorporating Genetic and Molecular Features
Predictive models often feature genetic and molecular data, like mutation information, to forecast drug efficacy and safety. Deep learning techniques analyze these molecular features in concert with pharmacogenomic data, enhancing the precision with which drug risks are identified. This approach strives to tailor drug administration strategies to individuals’ genetic makeup, thereby mitigating potential risks.
Leveraging Clinical and Pharmacogenomic Data
When it comes to personalizing medicine, incorporating clinical studies and pharmacogenomic interactions into predictive models is key. These data sources are instrumental in understanding how genetic factors influence drug response. Moreover, pharmacogenomic data provides critical insights into the optimal drug choice and dosing for each patient, based on their unique genetic profile.
Use of Real-World Data and Electronic Health Records
Lastly, real-world data derived from electronic health records (EHRs) serves as a rich resource for predictive models. Here, natural language processing (NLP) techniques are used to extract meaningful information from unstructured clinical narratives. This integration of real-world evidence with traditional data facilitates a comprehensive understanding of drug risks in diverse patient populations.
Specialized Predictive Models in Oncology
Precision oncology leverages specialized predictive models to tailor cancer treatment, enhance drug discovery, and mitigate risk. These models integrate various data sets, from genetic biomarkers to clinical trial outcomes, thus refining drug response prediction and aiding in the development of targeted therapies.
Targeting Cancer Treatment Through Predictive Models
Predictive models in oncology are pivotal for the identification of targeted therapies. These models allow researchers to effectively predict how certain cancers will respond to specific drugs, greatly impacting the successful outcomes in clinical trials. For instance, the utilization of machine learning approaches for drug response prediction in cancer has shown promising potential to enhance treatment personalization.
Advances in Precision Oncology and Biomarkers
Precision oncology has transformed cancer treatment through the use of biomarkers. These are biological indicators that help in predicting how a patient will respond to a particular therapy, leading to a more efficient drug discovery and development process. The assessment of biomarkers has become a cornerstone in developing novel therapeutic strategies, including combination therapy.
Innovations in Cancer Risk Prediction Models
Innovations in risk prediction models enable early detection of potential adverse events, including susceptibility to opioid use disorder in pain management post-treatment. These models are integral to understanding the varied risks associated with cancer and its treatment, contributing to more informed decision-making in patient care.
Multi-Omic Correlates in Cancer Therapies
The integration of multi-omic correlates—genes, proteins, and other molecular data—into predictive models has greatly enhanced the understanding of cancer biology. This comprehensive approach improves the prediction of drug efficacy and may inform the design of drug combination prediction models, offering insights into how different therapies can be effectively combined.
Utilizing Patient-Derived Models for Therapy Prediction
Patient-derived xenograft mouse models are increasingly used to anticipate how cancer patients will respond to treatments. These models, which involve transplanting human tumors into mice, provide a more accurate representation of drug response prediction in preclinical settings, helping to identify the most promising treatments for progression into clinical trials.
Predictive Models for Drug Repositioning and Combination Therapies
Predictive models are transforming the field of pharmacology, particularly in the realms of drug repurposing and combination therapies. By leveraging predictive analytics and machine learning techniques, researchers can identify new applications for existing drugs and anticipate the efficacy of drug combinations.
Drug Repurposing Using Predictive Analytics
The process of drug repurposing involves identifying new therapeutic potentials for existing drugs. Predictive analytics apply computational models that integrate diverse datasets to forecast new drug applications. For instance, autoencoders, a form of neural networks, can deduce chemical properties from vast chemical libraries, pinpointing candidates for repurposing with a higher likelihood of success.
Predicting Efficacy of Drug Combinations
The precision of drug combination prediction hinges on comprehending how drugs interact. Predictive models use cell line data and drug sensitivity prediction algorithms to establish which combinations might be most effective. This approach is pivotal for combination therapy design, where the objective is to maximize therapeutic effects while minimizing adverse interactions.
Utilizing High-Throughput Data for Combination Therapy
High-throughput screening generates extensive data that predictive models analyze to determine potential drug combinations. Through processing cell line data and other biological information, researchers can swiftly evaluate thousands of drug interactions to identify promising combination therapies for further investigation.
Role of Machine Learning in Drug Synergy Prediction
Machine learning is indispensable in predicting drug synergies, whereby the combined effect of drugs exceeds the sum of their individual effects. Machine learning algorithms analyze complex datasets to discern patterns and predict interactions, a task impractical for human analysis alone. Thus, machine learning accelerates the discovery of combination therapies that can be tailored to individual patient needs.
Frontiers and Future Directions
The exploration of predictive models in drug safety is rapidly advancing, bringing forth innovative techniques and integrative approaches. This evolution promises to enhance the precision of adverse event predictions and tailor drug safety assessments to individual patient profiles.
Emerging Techniques in Predictive Modeling
The field of predictive modeling is witnessing significant advancements through the adoption of deep learning and variational autoencoders. These techniques are especially proficient in decoding complex, high-dimensional biomedical data, leading to more accurate predictions of drug risks. Researchers are now leveraging self-learning algorithms to refine the identification of potential adverse drug reactions from existing databases.
Integrative Approaches to Drug Risk Prediction
To enhance the predictive power of models, scientists are combining statistical methods with machine learning algorithms. This integrative approach utilizes vast arrays of data from clinical trials and real-world evidence, increasing the reliability of predictions. It allows for a more comprehensive evaluation of FDA-approved drugs, integrating various data sources to anticipate and mitigate potential risks.
Potential of AI in Personalized Drug Safety
The potential for AI to drive personalized medicine in the realm of drug safety is immense. Predictive analytics are shifting towards patient-specific models, where individual genetic profiles and medical histories inform the safety profile of medications. This bespoke approach to medicine aims to minimize adverse events by foreseeing how different patients might react to certain drugs.
Regulatory Considerations and Model Validation
For predictive models to be effectively applied in clinical settings, they must undergo stringent validation processes to meet regulatory standards. Regulatory bodies are actively developing frameworks to evaluate the efficacy of predictive models in drug safety. This involves rigorous testing and validation of the models to ensure accuracy and consistency in adverse drug reaction predictions.
Frequently Asked Questions
Predictive modeling leverages historical data to foresee potential drug risks, thus enhancing patient safety. Each question below delves into aspects critical to understanding and improving predictive models in the realm of drug safety.
How can predictive modeling improve the identification of potential drug risks?
Predictive modeling applies algorithms and statistical techniques to analyze data on drug use and outcomes, enabling the early identification of adverse drug events. This approach can highlight risk factors that may not be evident through traditional analysis.
What types of data are most valuable when creating a predictive model for drug safety?
Data that is comprehensive and high-quality, including electronic health records, clinical trial data, and real-world evidence, is invaluable for creating robust predictive models. Detailed information on drug dosage, patient demographics, and prior health history contribute to a model’s accuracy.
What are the key factors that predictive models consider when assessing drug risks?
They typically consider variables such as patient age, genetics, medical history, polypharmacy, and drug interactions to assess the likelihood of adverse events. The chosen factors depend on the specific drug and the context of its use.
How do predictive models differentiate between correlation and causation in drug risk analysis?
Predictive models utilize statistical methods to identify patterns that suggest causal relationships while controlling for confounding variables. Cross-validation and other machine learning techniques can help to differentiate true causation from mere correlation.
What methodologies are commonly used in the development of predictive models for pharmacovigilance?
Methodologies such as multivariable logistic regression, machine learning algorithms, and cross-validated predictive modeling are employed. These approaches are designed to enhance model generalizability and minimize overfitting.
How effective have predictive models been in reducing adverse drug reactions in real-world settings?
Predictive models, when properly designed and applied, have demonstrated effectiveness in reducing the incidence of adverse drug events by alerting healthcare professionals to potential risks, thereby improving patient safety and outcomes.
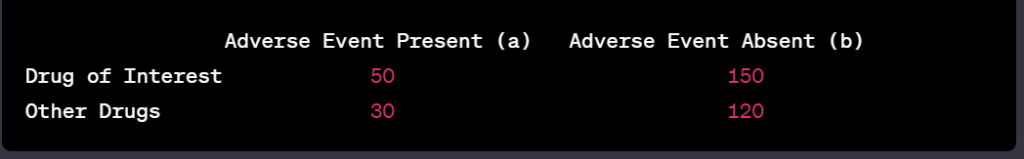
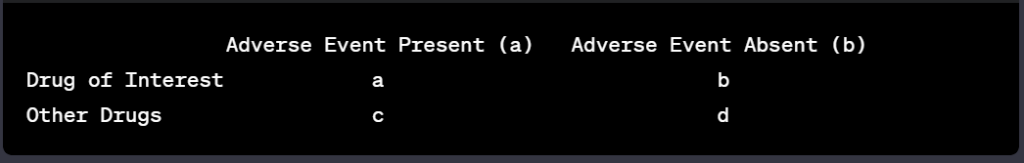
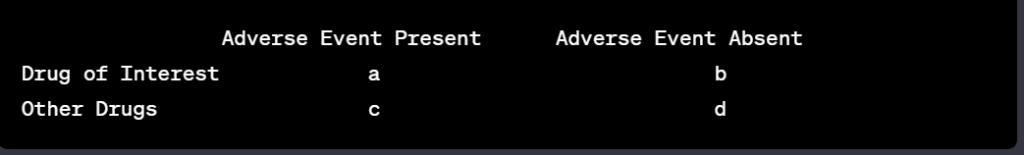



 Peter J Pitts, President of the Center for Medicine in the Public Interest, and Hervé Le Louet, President of CIOMS, have just published an intellectually-stimulating essay on the future of pharmacovigilance entitled “Advancing Drug Safety Through Prospective Pharmacovigilance“. The complete reference of the article is: Pitts PJ, Le Louet H. Ther Innov Regul Sci 2018;
Peter J Pitts, President of the Center for Medicine in the Public Interest, and Hervé Le Louet, President of CIOMS, have just published an intellectually-stimulating essay on the future of pharmacovigilance entitled “Advancing Drug Safety Through Prospective Pharmacovigilance“. The complete reference of the article is: Pitts PJ, Le Louet H. Ther Innov Regul Sci 2018;