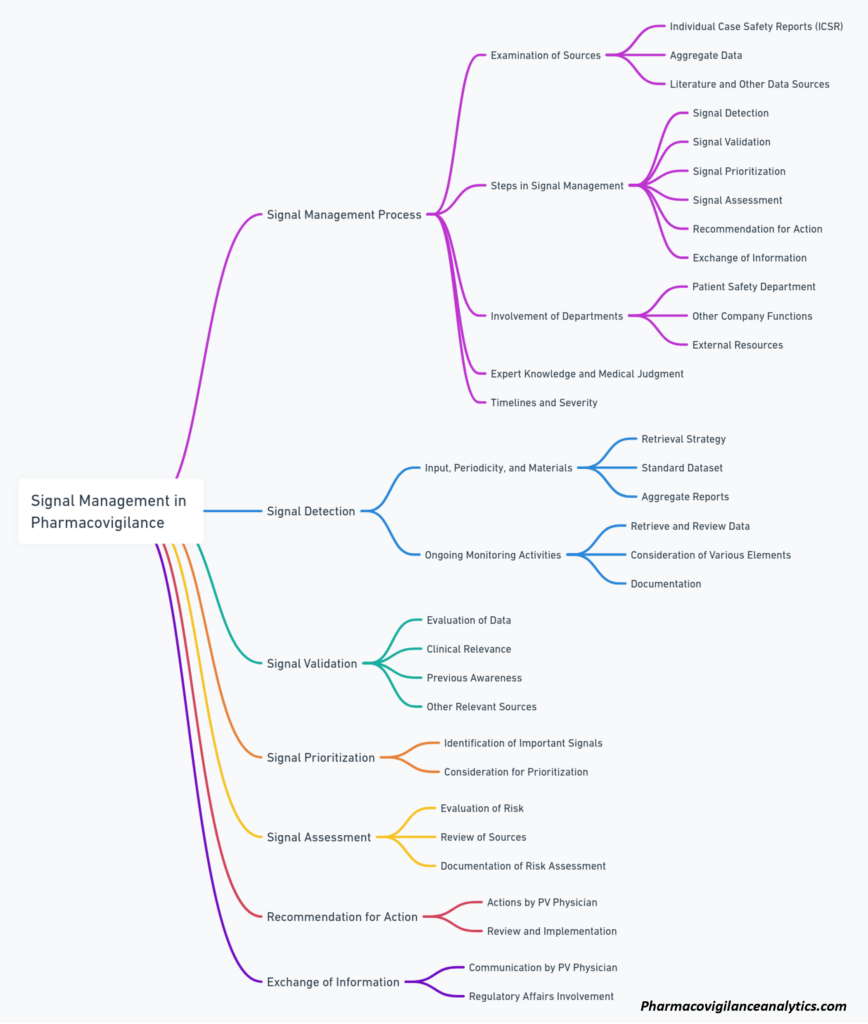
This mind map outlines the key components and steps involved in the signal management process in pharmacovigilance, including signal detection, validation, prioritization, assessment, and the exchange of information.
Enjoy!
Your best resource for PV analytics news, content and innovation!
[…] Signal management in pharmacovigilance is a critical aspect of ensuring drug safety and protecting public health. Its primary aim is to detect, prioritize, and evaluate potential safety signals related to medications, allowing for a better understanding of the benefits and risks associated with their use. By effectively managing these signals, healthcare professionals and regulatory authorities can implement appropriate measures to mitigate adverse drug events and optimize patient outcomes. […]